Abstract
IntroductionFLT3 mutations are found in up to 30% of AML patients (pts), with 80% being internal tandem duplications (ITD). Based largely on data in younger pts from an era prior to the use of FLT3 inhibitors or hypomethylating agents (HMA) and venetoclax (Ven), FLT3-ITD mutated (FLT3-ITD+) AML is associated with higher relapse rates and decreased survival compared to FLT3 wild-type (WT) disease. Post hoc analysis from the VIALE-A trial demonstrated comparable outcomes between pts with FLT3-mutated vsFLT3-WT AML treated with HMA+Ven. Further, the AMLSG16-10 trial demonstrated improved survival among pts aged 60-70 with FLT-ITD+ AML treated with "7+3” and midostaurin compared to historical controls. To determine the clinical significance of FLT3-ITD+ in older adults with AML, we assessed the prevalence of FLT3-ITD mutations in pts with AML age ≥ 60 years treated at Dana Farber Cancer Institute (DFCI), evaluated whether FLT3-ITD+ is associated with an inferior prognosis in this age group and identified other specific characteristics affecting survival.
Methods We included older (age ≥ 60) pts diagnosed with AML at DFCI between August 2015 and September 2021 and treated with: daunorubicin and cytarabine ("7+3") with or without a third drug; liposomal daunorubicin and cytarabine (CPX351); or HMA-based therapy
(HMA+/-Ven). Groups were defined by presence (FLT3-ITD+) or absence (FLT3-ITD-) of FLT3-ITD mutation. We excluded pts in whom diagnostic molecular analysis was not performed, those who had acute promyelocytic leukemia or who received other treatments. Overall survival (OS) was evaluated by the Kaplan-Meier method. Cox regression models were fitted to assess the effect of FLT3-ITD mutations and additional covariates (age, prior myeloid disease, cytogenetics, molecular groups, treatment, and transplant as time-dependent covariate) on OS.
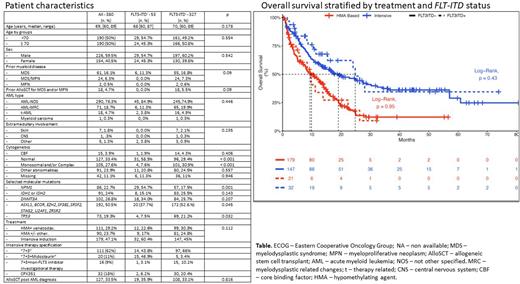
Results Of the 380 pts, 53 (14%) were FLT3-ITD+ and 327 (85%) FLT3-ITD-. The median age was 69 years (range 60-89) and was similar between groups (68 [range 60-87] vs 70 [range (60-89], respectively, p=0.18). Intensive chemotherapy (IC) was used in 179 pts: 111 (62%) with "7+3", 20 (11%) with "7+3"+midostaurin (15 [47%] in FLT-ITD+ and 5 [3%] in FLT-ITD- in pts with FLT-TKD mutation), 16 (9%) with "7+3"+other non-FLT3 inhibitor investigational therapy and 32 (18%) with CPX351. HMA+Ven or HMA was used in 111 (29%) and 90 (24%) of pts, respectively. Baseline characteristics in FLT3-ITD+ and FLT3-ITD- pts differed by rates of normal karyotype, monosomal and/or complex karyotype (both p<0.001), and specific molecular co-mutations: (TP53 [p=0.032], secondary type mutations [0.045] and NPM1 [p=0.001]. Table).
The median OS of the entire cohort was 13.6 months (CI 95% 11-16) and did not differ between pts with FLT3-ITD+vsFLT3-ITD- (median OS 15.4 [CI 95% 11-28] vs 13.1 [CI 95% 11-16], p=0.33). The median OS was similar between FLT3-ITD+vsFLT3-ITD- pts among those who received IC: 24.8 months (CI 95% 15-NA) vs 19.1 (CI 95% 13-30; p=0.43) and HMA-based-therapy: 9.33 months (CI 95% 6-NA) vs 9.9 (CI 95% 8-14; p=0.95), respectively (Figure). The addition of midostaurin could potentially ameliorate an adverse impact of a FLT3-ITD mutation. Thus, we conducted a sensitivity analysis in IC-treated pts who did not receive midostaurin, which demonstrated similar outcomes in FLT3-ITD+ ( n=17) vsFLT-ITD- (n=142): Median OS 15.4 months (CI 95% 4-NR) vs 17.5 (CI 95% 13-30), respectively, p=0.97. In a multivariable analysis ( MVA), FLT3-ITD status was not associated with OS (HR 1.1 [CI 95% 0.7-1.6] p=0.66).
Factors associated with OS in the MVA were: treatment with HMA+Ven vs HMA (HR 0.62 [CI 95% 0.4-0.9], p=0.01), age ≥ 70 years (HR 1.03 [CI 95% 1.003-1.1], p=0.03), monosomal and/or complex karyotype vs normal karyotype (HR 1.67 [CI 95% 1.2-2.4], p=0.009) and TP53 mutations (HR 1.63 [CI 95% 1.1-2.3], p=0.005). Of note, treatment with intensive therapy vs HMA was not associated with OS (0.98 [CI 95% 0.7-1.5], p=0.93).
Conclusion In older pts with AML, FLT3-ITD mutations were less common than reported in younger pts and were not associated with an adverse prognosis. Age ≥ 70 years, complex/ monosomal karyotype and presence of TP53 were associated with worse survival. Compared to HMA, therapy with HMA+Ven was associated with improved OS, while treatment with IC was not. The prognostic significance of a FLT3-ITD mutation should be re-evaluated in older pts with AML.
Disclosures
Winer:Novartis, Jazz Pharmaceuticals, Pfizer, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Takeda Pharmaceuticals: Consultancy; Curis: Consultancy. Garcia:AbbVie: Research Funding; AbbVie, Astellas, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; AbbVie, Genentech, AstraZeneca, Prelude and Pfizer: Other: Clinical trial support (institutional) , Research Funding. Luskin:Pfizer: Honoraria; Abbvie: Research Funding; Novartis: Research Funding. Stahl:Novartis: Other: Advisory Board; Curis Oncology: Consultancy; Boston Consulting: Consultancy. Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. DeAngelo:Abbvie: Research Funding; Pfizer: Consultancy; Blueprint Medicines Corporation: Consultancy; Incyte: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Agios: Consultancy; Amgen: Consultancy; Glycomimetics: Research Funding; Shire: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy. Stone:Novartis: Consultancy; Syntrix: Consultancy; Syndax: Consultancy; Kura Oncology: Consultancy; BMS: Consultancy; Innate: Consultancy; GSK: Consultancy; Epizyme: Consultancy; Boston Pharmaceuticals: Consultancy; Astellas: Consultancy; Arog: Consultancy, Research Funding; Aprea: Consultancy; Elevate Bio: Consultancy; Apteva: Consultancy; Abbvie: Consultancy, Research Funding; Janssen: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Foghorn Therapeutics: Consultancy; Gemoab: Consultancy; Actinium: Consultancy; OncoNova: Consultancy; Syros: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.